Resources
CRID Patient Information Sheet
 This is a MS Word template of the "CRID - Patient Information Sheet". You can customize and share this with your patient community.
This is a MS Word template of the "CRID - Patient Information Sheet". You can customize and share this with your patient community.
Click here to download the document.
Supporters
 A number of parent-led disease organizations are showing their support for using CRID unique patient IDs in rare disease research. These organizations recognize the importance of having a system
for identifying patients with rare diseases across research studies, which can facilitate data sharing, collaboration, and ultimately, accelerate the development of
effective treatments. Click HERE to view these..
A number of parent-led disease organizations are showing their support for using CRID unique patient IDs in rare disease research. These organizations recognize the importance of having a system
for identifying patients with rare diseases across research studies, which can facilitate data sharing, collaboration, and ultimately, accelerate the development of
effective treatments. Click HERE to view these..
CRID Format
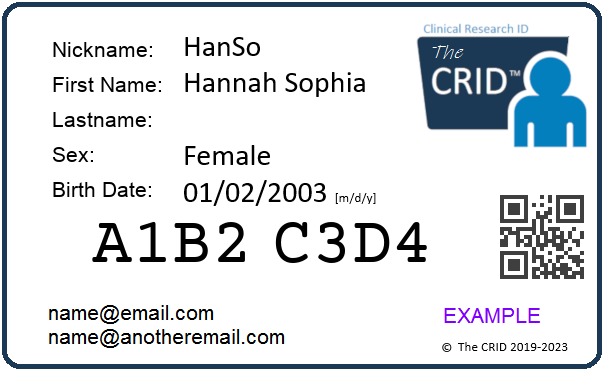 The CRID identifier is 8 characters long and is composed of alphanumeric characters (letters and numbers). It does not contain any spaces or any special characters.
The CRID identifier is 8 characters long and is composed of alphanumeric characters (letters and numbers). It does not contain any spaces or any special characters.
To avoid confusion, it does not use the numbers 1 or 0, or the letters I, O or L.
This allows for 852,891,037,441 unique identifiers.
Data Encryption & Privacy
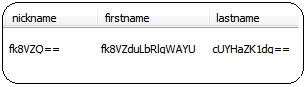 All personal data in the database is encrypted using the Advanced Encryption Standard (AES-128). It's also encrypted at-rest and in-transit.
The sole purpose of CRID is to "promote data sharing and eliminate data silos". We will never share or sell your information. We don't use 3rd party website tracking cookies, like Google Analytics.
The CRID system is installed on secure Amazon AWS servers located in Ireland (EU).
All personal data in the database is encrypted using the Advanced Encryption Standard (AES-128). It's also encrypted at-rest and in-transit.
The sole purpose of CRID is to "promote data sharing and eliminate data silos". We will never share or sell your information. We don't use 3rd party website tracking cookies, like Google Analytics.
The CRID system is installed on secure Amazon AWS servers located in Ireland (EU).
Validate a CRID
You can validate a CRID identifier (i.e. check that it exists!) by clicking HERE.
RESTful API
 CRID provides an API for use by authorized research organizations. This API enables researchers to interact with CRID programatically and embed CRID functionality in their applicatiions. Contact us for API code examples in Curl, PHP, JavaScript, Java and Python.
CRID provides an API for use by authorized research organizations. This API enables researchers to interact with CRID programatically and embed CRID functionality in their applicatiions. Contact us for API code examples in Curl, PHP, JavaScript, Java and Python.
API Endpoints include:
• Verify a CRID identifier• Get Study Information
• Get Token Information
• More to be added....
You will need to contact us in order to be granted access to the CRID API. Your research study must be approved by a recognized IRB.
Training & Webinars
News
Mar 7th, 2024
NR2F1 Foundation | Webinar | CRID with Gerry Nesbitt
Mar 1st, 2024
Creating a New Account is now a 2-step process to help ensure all email addresses are genuine.
Jan 16th, 2024
Cure GABA-A | Launch and Learn | CRID with Gerry Nesbitt
Sept 21st, 2022
CRID RESTful API Launched
Aug 23rd, 2022
CRID Training Video
Dec 2nd-6th, 2022
American Epilepsy Society Meeting (Nashville, TN)

